Periodicity
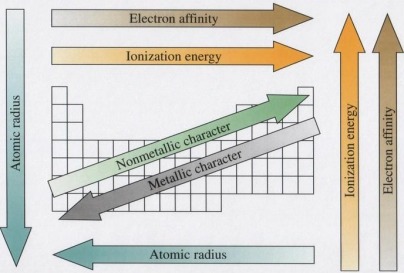
Physical Properties: The first ionization energy of element is defined as the energy required to remove one electron from an atom in its gaseous state. (This is measured in kJ mol-¹)
Example: X(g)--> X+(g) + e-
Electronegativity is the relative measure of the attraction that an atom has for a shared pair of electrons when it is covalently bonded to another atom. As the size of the atom decreases the electronegativity increases, so the value increases across a period and decreases down a group. The Three most electronegative elements are F, N and O.
The atomic radius is the distance from the nucleus to the outermost electron. The radius is usually defined as half the distance between the nuclei of two bonded atoms of the same element. The atomic radius decreases from left to right, bottom to top. In other words it descreases across a period and increases down a group. Across a period electrons are being added to the same energy level, but the number of protons in the nucleus increases. This attracts the energy level closer to the nucleus, this is why the atomic radius decreases across a period.
For ionic radius it is important to destinguish between positive ions (cations) and negative ions (anions). Both increase in size down a group as the outer level gets further from the nucleus. Cations contain less electrons than protons so the electrostatic attraction between the nucleus and the outermost electron is greater and the ion is smaller than the parent atom. It is also smaller because the number of electron shells has decreased by one. Anions contain more electrons than protons, therefore they are larger than the parent atom. Across a period the size decreases because the number of electrons remains the same but the number of protons increases.
The melting points depend both on the structure of the element and on the type of attractive forces holding the atoms together. Using period 3 as an example:
-At the left of the period elements exhibit metallic bonding (Na, Mg, Al) which increases in strength as the number of valence electrons increase. Silicon in the middle of the period has a macromolecular covalent structure with very strong bonds resulting in a very high melting point.
-Elements in group 5, 6, and 7 (P4, S8, and Cl2) show simple molecular structures with weak van der Waals' forces of attraction between the molecules. The noble gases (Ar) exist as monatomic molecules (single atoms) with extremely weak forces of attraction between the atoms.
Within groups:
-In group 1 the melting point decreases down the group as the atoms become larger and the length of the metallic bond decreases.
-In group 7 the van der Waals' attractive forces between the diatomic molecules increase down the group so the melting points increase.
Example: X(g)--> X+(g) + e-
Electronegativity is the relative measure of the attraction that an atom has for a shared pair of electrons when it is covalently bonded to another atom. As the size of the atom decreases the electronegativity increases, so the value increases across a period and decreases down a group. The Three most electronegative elements are F, N and O.
The atomic radius is the distance from the nucleus to the outermost electron. The radius is usually defined as half the distance between the nuclei of two bonded atoms of the same element. The atomic radius decreases from left to right, bottom to top. In other words it descreases across a period and increases down a group. Across a period electrons are being added to the same energy level, but the number of protons in the nucleus increases. This attracts the energy level closer to the nucleus, this is why the atomic radius decreases across a period.
For ionic radius it is important to destinguish between positive ions (cations) and negative ions (anions). Both increase in size down a group as the outer level gets further from the nucleus. Cations contain less electrons than protons so the electrostatic attraction between the nucleus and the outermost electron is greater and the ion is smaller than the parent atom. It is also smaller because the number of electron shells has decreased by one. Anions contain more electrons than protons, therefore they are larger than the parent atom. Across a period the size decreases because the number of electrons remains the same but the number of protons increases.
The melting points depend both on the structure of the element and on the type of attractive forces holding the atoms together. Using period 3 as an example:
-At the left of the period elements exhibit metallic bonding (Na, Mg, Al) which increases in strength as the number of valence electrons increase. Silicon in the middle of the period has a macromolecular covalent structure with very strong bonds resulting in a very high melting point.
-Elements in group 5, 6, and 7 (P4, S8, and Cl2) show simple molecular structures with weak van der Waals' forces of attraction between the molecules. The noble gases (Ar) exist as monatomic molecules (single atoms) with extremely weak forces of attraction between the atoms.
Within groups:
-In group 1 the melting point decreases down the group as the atoms become larger and the length of the metallic bond decreases.
-In group 7 the van der Waals' attractive forces between the diatomic molecules increase down the group so the melting points increase.
