Analytical Techniques and Principles of Spectroscopy
With the help of modern-day technology and proper laboratories, scientists are able to use these techniques in order to determine exact structural formulas, analyze different composition of compounds, and determine purity.
What is a spectrum?
A spectrum is produce when a light source (sunbeam, torch, laser etc) passes through a refracting prism (piece of glass, or a diffraction grating) and the light is bent through an angle that depends on the wavength of the light passing through. If the light wavelength is long ( for instance, red light, wavelength 700nm) it is not deviated as much as a short wavelength (e.g. blue light, wavelength 400nm). Hence, any source of light consisting of several different wavelengths may be separated and displayed on a screen or the different wavelengths may be detected electronically and displayed.
If the light source contains all possible wavelengths (e.g. white light) then a continuous spectrum results (eg a rainbow)
“Analytical chemistry is what analytical chemists do.”-C. N. Reilly (1925-1981)
Seven Stages of an Analytical Method
1. Conception of analytical method (birth).
2. Successful demonstration that the analytical method works.
3. Establishment of the analytical method’s capabilities.
4. Widespread acceptance of the analytical method.
5. Continued development of the analytical method leads to significant improvements.
6. New cycle through steps 3–5.
7. Analytical method can no longer compete with newer analytical methods (death).
Spectroscopy
http://www.polysep.ucla.edu/che212/Notes/polymer%20spectroscopy.pdf (Fundamentals of Spectroscopy)
What is a spectrum?
A spectrum is produce when a light source (sunbeam, torch, laser etc) passes through a refracting prism (piece of glass, or a diffraction grating) and the light is bent through an angle that depends on the wavength of the light passing through. If the light wavelength is long ( for instance, red light, wavelength 700nm) it is not deviated as much as a short wavelength (e.g. blue light, wavelength 400nm). Hence, any source of light consisting of several different wavelengths may be separated and displayed on a screen or the different wavelengths may be detected electronically and displayed.
If the light source contains all possible wavelengths (e.g. white light) then a continuous spectrum results (eg a rainbow)
“Analytical chemistry is what analytical chemists do.”-C. N. Reilly (1925-1981)
Seven Stages of an Analytical Method
1. Conception of analytical method (birth).
2. Successful demonstration that the analytical method works.
3. Establishment of the analytical method’s capabilities.
4. Widespread acceptance of the analytical method.
5. Continued development of the analytical method leads to significant improvements.
6. New cycle through steps 3–5.
7. Analytical method can no longer compete with newer analytical methods (death).
Spectroscopy
http://www.polysep.ucla.edu/che212/Notes/polymer%20spectroscopy.pdf (Fundamentals of Spectroscopy)
- Spectroscopy is the study of how electromagnetic radiation (e.g. light) interacts with matter.
- Electromagnetic radiation forms a wide ranging spectrum from radio - microwave - infrared - visible light - uv - x-rays - gamma rays.
- Light can be considered as energy packets called photons which have both the properties of a 'particle' or a transverse 'wave'.
- The relationship between the speed of light, wavelength of the radiation and the frequency of the photon is given by ...
- c = , = wavelength (m), = frequency (Hz = s-1), c = speed of light 3 x 108 ms-1
- The relationship between the energy of the photon and its wave frequency is given by Planck's Equation
- E = h, E = energy of a single photon (J), = h = Planck's Constant (6.63 x 10-34 JHz-1), = frequency (Hz)
- E is for one photon interacting with one atom, so you need to multiply by the Avogadro Constant (6.02 x 1023 mol-1) to get Jmol-1, and then divide by 1000 to get kJmol-1
- When atoms absorb energy e.g. in hot flames, high voltage discharge etc., they can become excited from their normal stable ground state (n=1 in the case of hydrogen), up to a higher 'energy level' state.
- When the excited atoms lose energy and return to the ground state, they emit electromagnetic radiation, usually in the infrared, visible or ultraviolet regions.
- The emitted light can be split and analysed into its constituent frequencies, using a prism or grating in a spectrometer, to produce an atomic emission spectrum of 'lines' of different colour.
- Its also possible for the reverse process to happen, so if light is passed through the atoms in their ground state, absorption of energy occurs at exactly the same frequencies as observed in the emission spectrum. This shows up as black lines against the coloured spectrum background and is known as the absorption spectrum.
- Both emission and absorption spectra can be used to identify elements from their 'finger print' pattern, and from the intensity of the 'signal' quantitative measurements can be made.
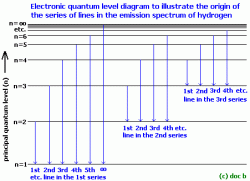
- Neils Bohr was the first scientist to successfully explain the spectrum of hydrogen using the theory of 'quantisation of energy' i.e. quantum theory.
- Atomic spectra are caused by electrons moving between energy levels (shells or sub-shells).
- When atom is excited, the electron 'jumps' to a higher electronic quantum level e.g. on absorption of a photon.
- This gives rise to absorption spectra.
- The atom 'relaxes' back to lower/ground electronic state and loses energy - emission of photons.
- This gives rise to emission spectra.
- The electron can only exist in certain definite energy (quantum) levels.
- For each atom a photon of light is absorbed or emitted, the electron changes from one level to another.
- The energy of the photon is the difference between the energies of the two quantised levels involved in the electronic change.
- e.g. E of photon = En=2 - En=1 for the 1st line in the 1st series of the hydrogen spectrum,
- where En=2 and En=1 are the specific energy values of the electron in the 1st and 2nd principal quantum levels.
- The frequency of the emitted or absorbed light is given by Planck's Equation: E = hv (details above)