Option C
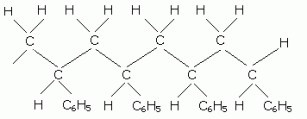
Addition Polymers:
Polymers are also divided into thermoplastics and thermosets. Most alkenes polymerize to form thermoplastics which can be remoulded each time they are heated. Thermostets cannot be softened or remoulded once they are formed and will be permanently destroyed if heated to a high temperature. Generrally the longer the chain length of a polymer the higher the melting point and strength. Apart from the length of the chain there are two other main structural features of addition polymers that affect their particular properties.
Branching-
Depending on the reaction conditions ethene can form high density or low density polythene. In high density poly(ethene), HDPE there is little branching. This gives long chains that can fit together closely making the polymer stronger, denser, and more rigid than low denstiy poly(ethene), LDPE. The presence of side chains in low density poly(ethene) results in a more resilient and flexible structure making it ideal for the production of film products, such as food wrappings.
In poly(ethene) the methyl groups can all have the same orientation along the polymer chain- isotactic. Due to the regular structure isotatic polymers are more crystalline and tough. Isotatic poly(ethene) is a thermoplastic and can be moulded into objects, such as car bumpers, and drawn into fibers for clothes and carpets. In atactic poly(propene) that chains are more loosely held so the polymer is soft and flexible, making it suitable for sealants and roofing materials.
Modifications:
Plasticized- Are small molecules that can fit between the long polymer chains. They weaken the attraction between the chains, acting as lubrificants, making the plastic more flexible. By varying the amount of plasticizer added PVC can form a complete range of polymers from rigid to fully pliable.
Volatile hydrocarbons- If pentane is added during the formation of polystyrene and the product heated in steam the pentane vaporizes producing expanded polystyrene. This light material is a good thermal insulator and is also used as packaging as it has good shock-absrbing properties.
The examples above illustrate how polymers can be tailor-made to perform a variety of functions based on properties such as strength, density, thermal and electrical insulation, flexibility, and lack of reactivity. The disadvantages are depletion of natural resources, disposal, and biodegradability.
The majority of polymers are carbon based. Currently oil is the major source of carbon although in the past it was coal. Both are fossil fuels and are in limited supply. Plastics have lack of reactivity so they are not easily disposed of. Particularly PVC and poly(propene), can be recycled and others are weakened and eventually decomposed by ultraviolet light. Plastics can be burned but if the temperature is not high enough poisonous dioxins can be produced along with toxic gases, such as hydrogen cyanide and hydrogen chloride. Most plastics do not occur naturally and are not degraded by micro-organisms. By incorporating natural polymers, such as starch, into plastics they can be made more biodegradeable. However, in the anaerobic conditions present in landfills biodegradation is very slow or will not occur at all.
Polymers are also divided into thermoplastics and thermosets. Most alkenes polymerize to form thermoplastics which can be remoulded each time they are heated. Thermostets cannot be softened or remoulded once they are formed and will be permanently destroyed if heated to a high temperature. Generrally the longer the chain length of a polymer the higher the melting point and strength. Apart from the length of the chain there are two other main structural features of addition polymers that affect their particular properties.
Branching-
Depending on the reaction conditions ethene can form high density or low density polythene. In high density poly(ethene), HDPE there is little branching. This gives long chains that can fit together closely making the polymer stronger, denser, and more rigid than low denstiy poly(ethene), LDPE. The presence of side chains in low density poly(ethene) results in a more resilient and flexible structure making it ideal for the production of film products, such as food wrappings.
In poly(ethene) the methyl groups can all have the same orientation along the polymer chain- isotactic. Due to the regular structure isotatic polymers are more crystalline and tough. Isotatic poly(ethene) is a thermoplastic and can be moulded into objects, such as car bumpers, and drawn into fibers for clothes and carpets. In atactic poly(propene) that chains are more loosely held so the polymer is soft and flexible, making it suitable for sealants and roofing materials.
Modifications:
Plasticized- Are small molecules that can fit between the long polymer chains. They weaken the attraction between the chains, acting as lubrificants, making the plastic more flexible. By varying the amount of plasticizer added PVC can form a complete range of polymers from rigid to fully pliable.
Volatile hydrocarbons- If pentane is added during the formation of polystyrene and the product heated in steam the pentane vaporizes producing expanded polystyrene. This light material is a good thermal insulator and is also used as packaging as it has good shock-absrbing properties.
The examples above illustrate how polymers can be tailor-made to perform a variety of functions based on properties such as strength, density, thermal and electrical insulation, flexibility, and lack of reactivity. The disadvantages are depletion of natural resources, disposal, and biodegradability.
The majority of polymers are carbon based. Currently oil is the major source of carbon although in the past it was coal. Both are fossil fuels and are in limited supply. Plastics have lack of reactivity so they are not easily disposed of. Particularly PVC and poly(propene), can be recycled and others are weakened and eventually decomposed by ultraviolet light. Plastics can be burned but if the temperature is not high enough poisonous dioxins can be produced along with toxic gases, such as hydrogen cyanide and hydrogen chloride. Most plastics do not occur naturally and are not degraded by micro-organisms. By incorporating natural polymers, such as starch, into plastics they can be made more biodegradeable. However, in the anaerobic conditions present in landfills biodegradation is very slow or will not occur at all.
