1.4 Mass and gaseous volume relationships in chemical equations
1.4.1: Calculate theoretical yields from chemical equations
Ex: CH4(g) + 2O2(g) --> CO2(g) + 2H2O(l)
What volume of CO2 is produced when 180g of CH4 burns?
16gCH4__________22.4dm3 x 1CO2
180g____________ X
X= 252dm3 of CO2 is produced
1.4.2 : Determine the limiting reactant and the reactant in excess when quantities of reacting substance are given.
- The reactant is said to be in excess(RE) ( there is too much of this reactant)
- The other reactant limits how much product will be made. Once it runs out, the reaction stops. This is called limiting reactant (RL)
Ex: How many grams of NO are produced if 4 moles of NH3 are burned in 20mol of O2?
2NH3 + O2 --> 2NO + 3H2
moles available: n2NH3 = 4 moles
nO2 = 20 moles
moles required: 2NH3_______O2 2NH3________O2
4_________X X__________20
X = 2 O2 X = 40 NH3
NH3 is the limiting reactant since less moles are available than required
1.4.3 : Solve problems involving theoretical, experimental and percentage yield
Percentage Yield= (Experimental /Expected) x 100
1.4.4 : Apply Avogadro's law to calculate reacting volumes of gases
1.4.5 : Apply concept of molar volume at standard temperature and pressure (STP) in calculations
Temperature: 273 K
Pressure: 2.24 x 10 -2 m3mol (22.4dm3mol-1) or 101.3KPa
1.4.6 : Solve problems involving the relationship betwen temperature, pressure and volume for a fized mass of an ideal gas
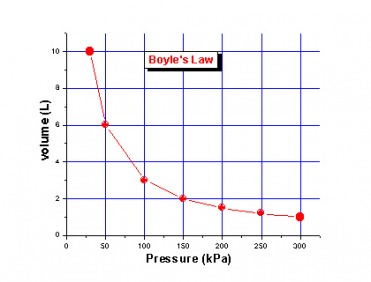
Boyle's Law
Pressure in inversely proportional to volume, while time is constant
P1V1=P2V2
Ex: CH4(g) + 2O2(g) --> CO2(g) + 2H2O(l)
What volume of CO2 is produced when 180g of CH4 burns?
16gCH4__________22.4dm3 x 1CO2
180g____________ X
X= 252dm3 of CO2 is produced
1.4.2 : Determine the limiting reactant and the reactant in excess when quantities of reacting substance are given.
- The reactant is said to be in excess(RE) ( there is too much of this reactant)
- The other reactant limits how much product will be made. Once it runs out, the reaction stops. This is called limiting reactant (RL)
Ex: How many grams of NO are produced if 4 moles of NH3 are burned in 20mol of O2?
2NH3 + O2 --> 2NO + 3H2
moles available: n2NH3 = 4 moles
nO2 = 20 moles
moles required: 2NH3_______O2 2NH3________O2
4_________X X__________20
X = 2 O2 X = 40 NH3
NH3 is the limiting reactant since less moles are available than required
1.4.3 : Solve problems involving theoretical, experimental and percentage yield
Percentage Yield= (Experimental /Expected) x 100
1.4.4 : Apply Avogadro's law to calculate reacting volumes of gases
1.4.5 : Apply concept of molar volume at standard temperature and pressure (STP) in calculations
Temperature: 273 K
Pressure: 2.24 x 10 -2 m3mol (22.4dm3mol-1) or 101.3KPa
1.4.6 : Solve problems involving the relationship betwen temperature, pressure and volume for a fized mass of an ideal gas
Boyle's Law
Pressure in inversely proportional to volume, while time is constant
P1V1=P2V2
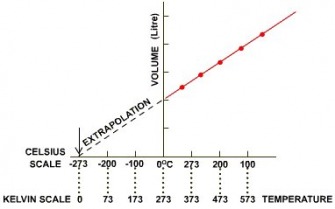
Charle's Law
Temperature needs to be in Kelvin ( graph need to be extrapolated)
Volume is proportional to temperature
Temperature needs to be in Kelvin ( graph need to be extrapolated)
Volume is proportional to temperature
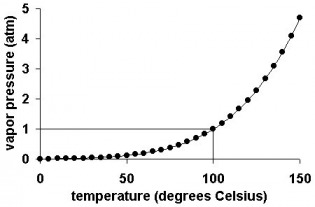
Gay Lassac's Law
Pressure is proportional to temperature
Pressure is proportional to temperature
Combined Laws : (P1V1)/T1=(P2V2)/T2
1.4.7 : Solve problems involving the ideal gas equation, PV=nRT
R-> 62.36 P mmHg
0.0821P atm
8.314 P KPa
Ex: What volume will 25g of O2 occupy at 20 degrees Celsius and pressure of 0.88atm?
nO2= 25/32= 0.78mols
PV= nRT V=(nRT)/P V=(0.78x0.0821x293)/0.88= 21.32dm3L-1
1.4.8: Analyse graphs relating to the ideal gas equation
1.4.7 : Solve problems involving the ideal gas equation, PV=nRT
R-> 62.36 P mmHg
0.0821P atm
8.314 P KPa
Ex: What volume will 25g of O2 occupy at 20 degrees Celsius and pressure of 0.88atm?
nO2= 25/32= 0.78mols
PV= nRT V=(nRT)/P V=(0.78x0.0821x293)/0.88= 21.32dm3L-1
1.4.8: Analyse graphs relating to the ideal gas equation
| chemical_reactionsstoichiometry.ppt |